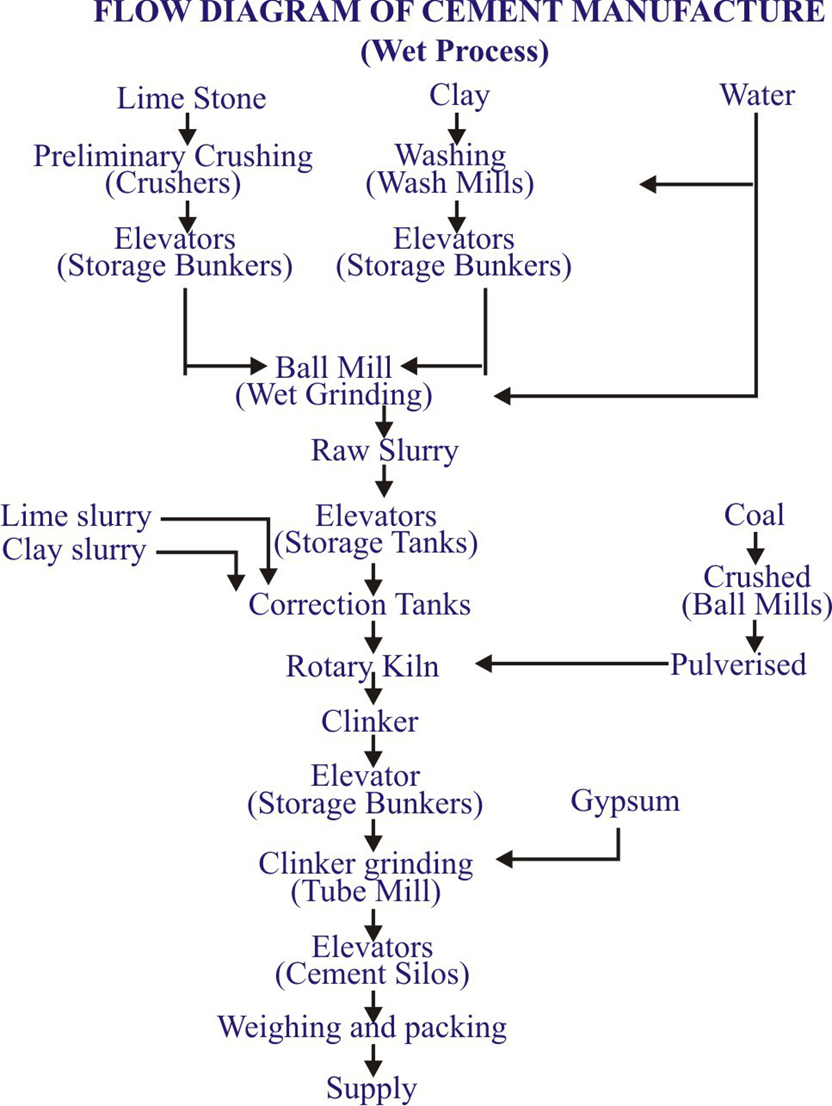
Raw materials for cement

The origins of concrete can be traced back to the Pantheon built by the ancient Romans some 2,000 years ago. The Pantheon’s foundations, walls and dome were all constructed using concrete made from pozzolan. However, with the technological knowledge available at the time, people could not yet understand why pozzolan provided such stone-like strength.
It was not until 1756 that British engineer John Smeaton, whilst investigating the hardening properties of certain limes in water, discovered that to obtain hydraulic lime, it was necessary to burn limestone containing clay (the primary raw materials for modern cement are also clay and limestone). This significant discovery laid the theoretical foundation for the development and advancement of modern cement. In 1824, British bricklayer Joseph Aspdin invented cement and secured the patent for Portland cement. Using limestone and clay as raw materials, he blended them in specific proportions before calcining the mixture in a vertical kiln similar to those used for burning lime. The resulting clinker was then ground into cement. Named Portland cement because its hardened colour resembled the building stone from Portland in England, it possessed excellent construction properties and marked an epoch-making milestone in cement history. Throughout the 20th century, while continually enhancing Portland cement’s properties, researchers successfully developed a range of cements tailored for specialised construction projects, such as high-alumina cement and speciality cements. The global variety of cement types has now expanded to over 100, with annual cement production reaching approximately 2 billion tonnes by 2007.
The product obtained by proportionately blending limestone and clay, followed by calcination and grinding, is silicate cement. However, with advancing research into cement, certain properties can be enhanced by incorporating specific quantities of blending materials (Ground Granulated Blast-furnace Slag, fly ash) and appropriate amounts of gypsum into silicate cement. The addition of gypsum prevents instantaneous setting of the cement. Consequently, pure silicate cement is generally referred to as clinker. The author estimates that, barring those employed within the cement industry, the vast majority of individuals would have little, if any, opportunity to encounter pure clinker.
Clinker + admixture (6%-15%) + gypsum (1.8%-2.5%) = ordinary silicate cement.
The most widely used cement in ready-mixed concrete is P▪O 42.5 cement, i.e., ordinary Portland cement achieving a compressive strength of 42.5 MPa at 28 days. Due to the minimal proportion of blended materials, its properties remain largely identical to those of Portland cement, with slight variations primarily manifested as: Slightly lower early strength; Enhanced corrosion resistance and heat resistance; slightly reduced hydration heat; and slightly diminished freeze-thaw resistance, abrasion resistance, and carbonation resistance.
Global cement manufacturing accounts for approximately 8% of worldwide carbon dioxide emissions. Consequently, reducing carbon emissions has become a key focus for industry research and development. Curbing carbon emissions entails lowering energy consumption; for instance, incorporating grinding aids during cement grinding effectively reduces energy usage while enhancing cement quality. SIDLEYchem AGA 2180 cement grinding aid significantly reduces energy consumption and has gained widespread recognition from customers.
Tricalcium silicate, dicalcium silicate and water react to form hydrated calcium silicate. It is easier to understand. The reaction process of tricalcium aluminates and tetracalcium ferroaluminate in the presence of gypsum is more complicated. I will use a diagram to introduce it. In short, a variety of hydrates are produced. Cement hydration reaction is a continuous process, which can be divided into five stages: 1. induction period; 2. acceleration period; 3. decay period; 4 stability period.
In the pre-induction period, the cement reacts sharply, and tricalcium silicate (C3S) dissolves in water and hardens rapidly, so the first exothermic peak occurs, and the time is very short, ending in less than 15min. After the induction period reaction is extremely slow, also known as the static period. Generally lasts 1-4 hours. It is the reason why the silicate cement paste can maintain plasticity within a few hours. The reasons for the stationary period are the protective layer theory and the delayed nucleation theory. The rapid production of hydrated tricalcium silicate during the pre-induction period covers the surface of the cement particles preventing further contact between water and cement. The time of initial setting is essentially equivalent to the end of the induction period.
The acceleration phase reaction re-intensifies as cement particles in the quiescent state become exposed once more to water molecules due to the rupture of their protective film (calcium silicate hydrate). A second exothermic peak emerges, and this stage concludes upon reaching its apex, typically within 4 to 8 hours. By this stage, final setting has concluded and hardening commences. The delayed nucleation theory posits that upon contact with water, tricalcium silicate undergoes rapid hydrolysis, releasing calcium ions and hydroxide ions into solution. This transforms the original tricalcium silicate surface into a ‘calcium-deficient’ or ‘silica-rich’ layer. Calcium ions in the liquid phase then chemically adsorb onto this silica-rich surface, imparting a positive charge. The high calcium ion concentration on the tricalcium silicate surface inhibits further cement hydrolysis, establishing a quiescent induction phase. Calcium and hydroxide ions subsequently dissolve at a low rate. When the liquid phase becomes supersaturated relative to calcium hydroxide, calcium hydroxide crystal nuclei rapidly form. As calcium hydroxide crystals grow, they reduce the concentration of calcium ions and hydroxide ions in solution, thereby resuming the accelerated hydration phase. The protective layer theory and delayed nucleation theory remain subjects of ongoing debate.
The decay phase, in which the reaction rate decreases with time, is due to the fact that the hydration of tricalcium silicate (C3S) is impeded as the hydrate accumulates around the cement particles, and thus the hydration moves from an accelerated process to a decelerated one. It lasts for about 12-24 hours and hydration is gradually controlled by the rate of diffusion.
In the steady state, the reaction rate is very low, the initial product, hydrated calcium silicate, grows on the surface of the cement particles, after which the water molecules need to pass through this layer of hydrated calcium silicate to enter the interior of the particles to continue the reaction with the unhydrated tricalcium silicate. The hydration reaction is completely controlled by the rate of diffusion.
The final hardened cement paste is a non-homogeneous multi-phase system consisting of a solid phase composed of various hydration products and unhydrated clinker, voids, water and air present in the voids. That is, the hardened cement paste is a porous body coexisting with solid, liquid and air phases. The relative contents of the various hydration products are: 70% C-S-H gel (hydrated calcium silicate), 20% Ca(OH)2, about 7% calcite and monosulphide-type hydrated calcium sulphoaluminate, and about 3% incompletely hydrated residual clinker and other trace components.
The process of reaction of tricalcium aluminate and tetracalcium ferroaluminate with the addition of gypsum is more complicated compared to the hydration process of calcium silicate. The reaction mechanism of cement can help us to deeply understand the plasticity property of concrete, the principle of slump loss, the initial setting time, and concrete cracking. The principles of concrete admixtures also revolve around the hydration process of cement. For example, the water reduction mechanism of superplasticiser is that polycarboxylate molecules are adsorbed on the surface of cement particles, preventing the cement particles from contacting each other by using charge repulsion and spatial site resistance, and releasing water molecules flocculated between cement particles. The theory of retarder is that the retarder molecules adsorbed on the surface of cement particles complex with calcium ions to hinder the formation of calcium hydroxide.




















