Surface treatment cellulose ether
Agglomeration and Lumping Phenomena: When untreated cellulose ether powder is directly poured into water, water molecules rapidly envelop the powder’s outer layer, preventing internal particles from effectively contacting water and readily forming lumping.
Left video: When ordinary cellulose ether is added to a solution, it forms clumps that require 3-6 hours of stirring to fully dissolve, wasting significant time and energy.
To address the agglomeration of cellulose ether, we have introduced surface-treated products: HPMC D905.
For certain water-based coatings or cleaning products, cellulose ether must be added directly to cold water for use. For such applications, cellulose ether is commonly surface-treated with glyoxal to enhance its delayed solubility:
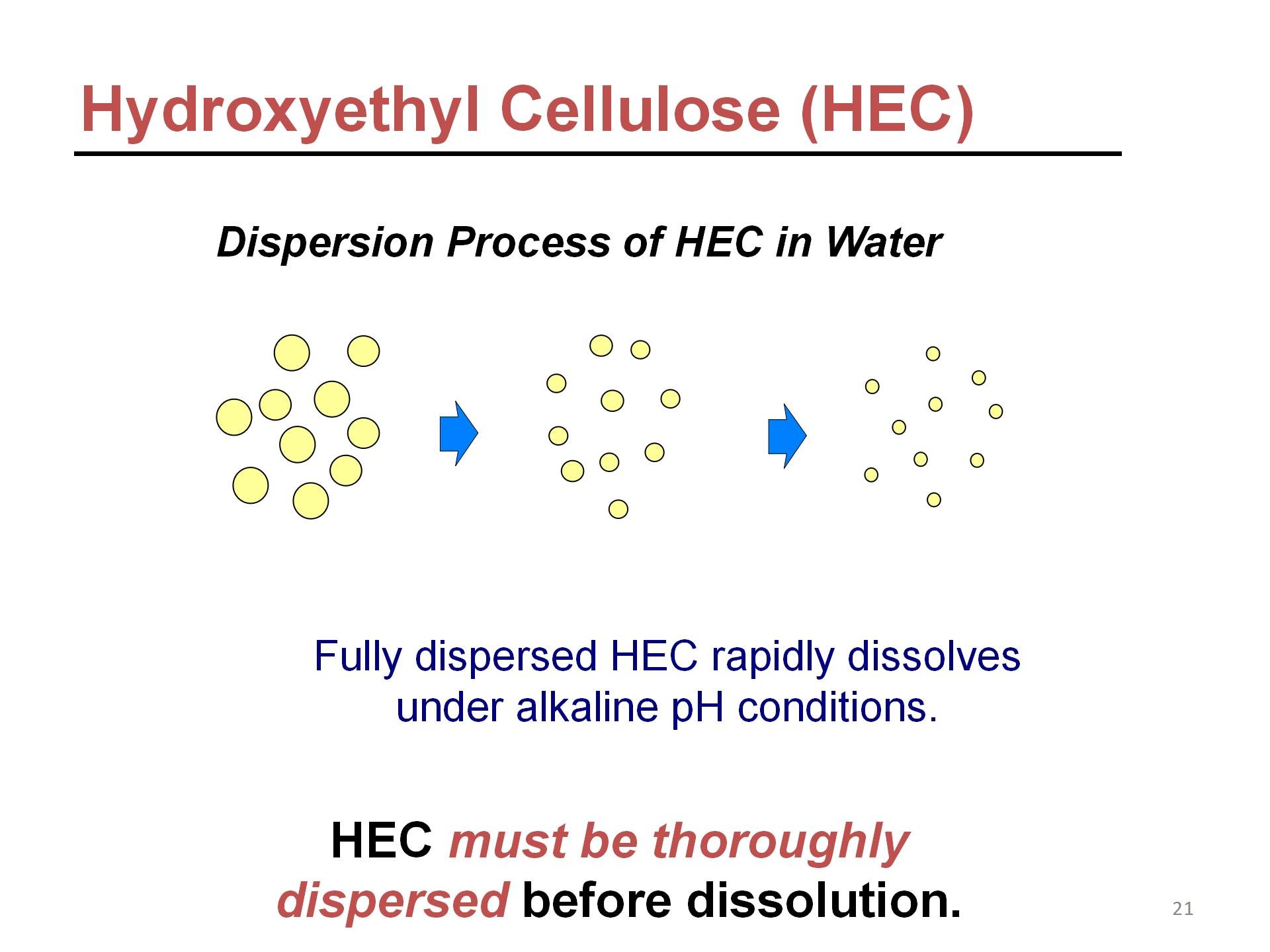
Glyoxal reacts with cellulose ether to form hemiacetal, moderately cross-linking the cellulose ether. This cross-linked product is insoluble in water but exhibits good dispersibility. During the hydration phase—the process of dispersing in water—only a minimal amount of cellulose ether is swelled.
Simultaneously, the hemiacetal structure begins to hydrolyze at a steady rate until the cross-linked bonds break. The cellulose ether then rapidly dissolves, causing a sharp increase in viscosity.
The hydrolysis rate increases with rising temperature and pH. In alkaline conditions with pH above 7, the delayed dissolution effect nearly disappears entirely. At this point, the cellulose ether fully dissolves and swells, forming a transparent, viscous cellulose ether solution.
During the final stages of painting and cosmetic production, the pH is typically adjusted to alkaline conditions. At this point, cellulose ether fully releases its viscosity, achieving the required product consistency. If full viscosity is needed earlier, simply adjust the pH of the cellulose ether dispersion to alkaline and allow the solution to swell for 1-2 hours to obtain a fully dissolved cellulose ether solution.
If adding cellulose ether during the final stage of paint or detergent preparation, first thoroughly wet the cellulose ether with sufficient water before incorporating it into the paint or detergent solution.